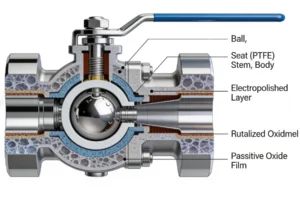
The pharmaceutical industry demands uncompromising standards for product purity, process safety, and regulatory compliance. Even the smallest trace of contamination—from valve materials, dead zones, or inadequate cleaning—can lead to batch rejection, costly recalls, or risks to patient health. Among industrial valves, 3-piece stainless steel ball valves have emerged as the gold standard for pharmaceutical applications, thanks to their sanitary design, modular construction, and compatibility with Clean-in-Place (CIP) and Sterilize-in-Place (SIP) processes.
Unlike one-piece or two-piece ball valves, the 3-piece design allows full disassembly of the valve body without removing it from the pipeline—simplifying maintenance, reducing downtime, and ensuring thorough cleaning. When constructed from high-grade stainless steel (e.g., 316L, 316Ti) and optimized for sanitary performance, these valves meet the strictest requirements of FDA, EMA, GMP, and 3A standards. This article explores the technical nuances of 3-piece Stainless Steel Ball Valves for pharma use, including regulatory compliance, sanitary design principles, material selection, technical specifications, manufacturing processes, real-world applications, maintenance best practices, and TIANYU’s tailored solutions—all aligned with the unique needs of pharmaceutical production.
I. Regulatory and Sanitary Requirements in Pharmaceutical Manufacturing
Pharmaceutical manufacturing is governed by rigorous global regulations that dictate every aspect of equipment design, including valves. Compliance is non-negotiable, as it directly impacts product quality and patient safety.

A. Key Global Regulations
- FDA (U.S. Food and Drug Administration): 21 CFR Part 177 specifies that materials in contact with food/drugs must be “safe for intended use.” For valves, this means no leaching of heavy metals (lead ≤5 μg/L, mercury ≤1 μg/L) or toxic additives. 21 CFR Part 211 (Current Good Manufacturing Practices, cGMP) requires equipment to be “designed, constructed, and maintained to facilitate cleaning, sanitization, and inspection.”
- EMA (European Medicines Agency): EudraLex Volume 4 (GMP) mandates that equipment must be “inert, non-reactive, non-absorptive, and non-contaminating.” Valves must be validated for CIP/SIP compatibility and undergo regular cleaning verification.
- 3A Sanitary Standards: Developed by the 3-A Sanitary Standards, Inc., these standards (e.g., 3A 603-02 for ball valves) define design criteria for sanitary equipment, including surface finish, flow channel geometry, and sealing materials.
- ISO Standards: ISO 14644 (cleanroom classification) and ISO 10993 (biological evaluation of medical devices) provide additional guidelines for valve use in sterile manufacturing.
B. Sanitary Performance Requirements
Beyond regulatory compliance, pharmaceutical valves must meet specific performance benchmarks to ensure product purity:
- No Dead Zones: Flow channels must be smooth and free of crevices, threads, or recesses where product residue or microorganisms can accumulate. Dead volume (unflushed areas) must be ≤0.1 mL per inch of nominal diameter (DN).
- Surface Finish: Contact surfaces (valve body, ball, stem) must have a Ra (arithmetic mean roughness) of ≤0.38 μm (15 microinches) for sterile applications—achieved via electrolytic polishing. This smooth finish minimizes product adhesion and microbial growth.
- CIP/SIP Compatibility: Valves must withstand repeated CIP (80–95℃, alkaline/acidic detergents) and SIP (121–134℃, steam under pressure) cycles without material degradation.
- Leak-Tight Sealing: Zero external leakage (to prevent environmental contamination) and minimal internal leakage (≤0.1 cm³/min for liquid service, per ISO 5208 Class VI) to avoid cross-contamination between batches.
- Microbial Control: After SIP, valves must achieve a sterility assurance level (SAL) of 10⁻⁶ (one viable microorganism per million sterilization cycles).
C. Consequences of Non-Compliance
Failure to meet these requirements can have severe consequences:
- Batch rejection: A single contaminated batch can cost \(500,000–\)2 million in lost production.
- Regulatory fines: The FDA can impose fines of up to $250,000 per violation for cGMP non-compliance.
- Reputational damage: Product recalls erode customer trust and market share—e.g., a 2022 recall of a common antibiotic due to valve-related contamination affected 1.2 million doses.
II. 3-Piece Design: Core Advantages for Pharmaceutical Applications
The 3-piece ball valve’s modular construction—consisting of two end caps, a central body, and a removable ball/stem assembly—addresses the unique challenges of pharmaceutical manufacturing better than one-piece or two-piece designs.
A. Modular Construction and Maintenance Efficiency
- Disassembly Without Pipeline Removal: Unlike two-piece valves (which require full pipeline removal for maintenance) or one-piece valves (non-serviceable), 3-piece valves allow end caps to be unbolted, and the ball/stem assembly to be extracted while the valve remains flanged to the pipeline. This reduces maintenance time by 60%—critical for pharmaceutical plants where downtime can cost \(10,000–\)50,000 per hour.
- Thorough Cleaning Access: Full disassembly exposes all contact surfaces to manual cleaning (if CIP is insufficient), ensuring no residue remains in hard-to-reach areas. For example, in API (Active Pharmaceutical Ingredient) production, where sticky or viscous products can adhere to valve internals, this access is invaluable.
- Component Replacement: Individual parts (seals, ball, stem) can be replaced without replacing the entire valve—reducing maintenance costs by 40% over the valve’s lifecycle (typically 10–15 years).
B. Flow Channel Optimization for Sanitary Performance
3-piece stainless steel ball valves are engineered with flow channels that prioritize product purity:
- Full Bore Design: The ball’s bore matches the pipeline DN (e.g., DN50 valve → 50 mm bore) to minimize flow restriction and dead zones. This design ensures uniform CIP/SIP fluid distribution, with no areas where detergent or steam cannot reach.
- Smooth Transitions: Flow channels have rounded edges (radius ≥1.5 mm) and no sharp corners—preventing product buildup. The internal surface of the central body is electrolytically polished to Ra ≤0.38 μm, matching the ball and stem finish.
- No Threads in Contact Zones: End caps are bolted (not threaded) to the central body, eliminating thread crevices that trap residue. Threads for bonnet bolts are located outside the product contact area.
C. Versatility Across Pharmaceutical Processes
The 3-piece design adapts to diverse pharmaceutical applications:
- API Manufacturing: Handles viscous intermediates, solvents (e.g., ethanol, methanol), and reactive chemicals (e.g., acids, bases) with corrosion-resistant materials.
- Formulation and Filling: Provides precise flow control for liquid formulations (e.g., syrups, injectables) and maintains sterility during filling operations.
- Sterile Water Systems: Compatible with Purified Water (PW) and Water for Injection (WFI) systems, where high purity and CIP/SIP compatibility are critical.
- Biopharmaceutical Production: Meets the ultra-sanitary requirements of bioprocessing (e.g., cell culture media, protein purification) with smooth surfaces and low-shedding materials.
III. Material Selection: Stainless Steel and Sanitary Compounds
Material choice is critical for pharmaceutical valves—materials must be inert, corrosion-resistant, and non-contaminating. 3-piece ball valves rely on high-grade stainless steel for structural components and FDA-compliant elastomers for sealing.

A. Stainless Steel Alloys: 316L, 316Ti, and Beyond
Stainless steel is the material of choice for pharmaceutical valves due to its corrosion resistance, biocompatibility, and ability to achieve the required surface finish. The most common alloys are:
- 316L Stainless Steel: The industry standard for pharmaceutical applications. Key properties:
-
- Chemical composition (per ASTM A240): 16–18% Cr, 10–14% Ni, 2–3% Mo, ≤0.03% C.
-
- Pitting Resistance Equivalent Number (PREN): 25–30—resists corrosion from acids, bases, and chloride-containing detergents.
-
- Corrosion rate: ≤0.01 mm/year in typical pharmaceutical media (e.g., 10% HCl, 5% NaOH).
-
- Biocompatibility: Meets FDA 21 CFR Part 177.2600—no heavy metal leaching (lead ≤5 μg/L, nickel ≤10 μg/L).
- 316Ti Stainless Steel: A premium alloy for high-temperature or aggressive applications:
-
- Adds 0.2–0.7% titanium to 316L, which stabilizes carbides and prevents intergranular corrosion during welding or SIP (134℃).
-
- PREN: 28–32—superior to 316L in corrosive environments (e.g., WFI systems with high chloride levels).
- Hastelloy C-276: Used for extreme applications (e.g., strong acids like sulfuric acid or hydrochloric acid):
-
- PREN: 60–65—virtually immune to pitting and crevice corrosion.
-
- Biocompatibility: Meets ISO 10993-1—safe for contact with pharmaceutical products.
B. Sealing Materials: FDA-Compliant Elastomers and Polymers
Seals are critical for preventing leakage and maintaining sterility. Only FDA-approved materials are used:
- EPDM (Ethylene Propylene Diene Monomer): The most common seal material for pharmaceutical valves:
-
- Temperature range: -40℃ to 135℃—compatible with CIP (95℃) and SIP (121℃).
-
- Chemical resistance: Resists alkaline detergents, acids, and steam—ideal for general pharmaceutical use.
-
- Biocompatibility: Meets FDA 21 CFR Part 177.2600 and 3A 603-02.
- PTFE (Polytetrafluoroethylene): Used for aggressive media or high-temperature applications:
-
- Temperature range: -200℃ to 260℃—suitable for SIP up to 134℃.
-
- Chemical resistance: Inert to nearly all pharmaceutical chemicals, including strong acids and solvents.
-
- Limitation: Less flexible than EPDM—requires precise machining to ensure leak-tight sealing.
- FKM (Fluorocarbon Rubber): Used for high-temperature, oil-resistant applications:
-
- Temperature range: -20℃ to 200℃—compatible with high-temperature SIP (134℃) and oily formulations.
-
- Chemical resistance: Resists oils, greases, and aromatic solvents.
C. Material Compatibility Testing
Before use, all materials undergo rigorous compatibility testing to ensure they do not interact with pharmaceutical products or cleaning agents:
- Extractable/Leachable Testing: Per FDA 21 CFR Part 211.84(d), materials are soaked in simulated product media (e.g., water, ethanol) at 40℃ for 72 hours. Extractables (metals, organics) are measured via GC-MS and ICP-OES—total extractables must be ≤10 μg/mL.
- SIP Aging Testing: Seals are subjected to 1,000 SIP cycles (121℃, 30 minutes) to verify no degradation (swelling ≤5%, hardness change ≤10 Shore A).
- Microbial Growth Testing: Materials are inoculated with common pharmaceutical contaminants (e.g., E. coli, Staphylococcus aureus) and incubated for 24 hours—no microbial growth is allowed on contact surfaces.
IV. Sanitary Design Details: Beyond Basic Compliance
3-piece stainless steel ball valves for pharmaceutical use incorporate specialized design features to meet the industry’s ultra-sanitary requirements—going beyond basic regulatory compliance to minimize contamination risks.

A. Surface Finish and Preparation
- Electrolytic Polishing: Contact surfaces are electrolytically polished after mechanical polishing. This process removes surface imperfections, creates a passive chromium oxide layer (0.005–0.01 mm thick), and achieves a Ra ≤0.38 μm finish. Electrolytic polishing also reduces the surface area available for microbial adhesion by 40% compared to mechanical polishing alone.
- Passivation: After polishing, valves undergo nitric acid passivation (20% HNO₃, 40℃, 30 minutes) to enhance corrosion resistance. Passivation removes free iron from the surface, ensuring the chromium oxide layer is uniform and stable.
- Cleanroom Inspection: Surface finish is verified using a profilometer (Ra measurement) and visual inspection under 10x magnification—no scratches, pits, or tool marks are allowed.
B. Flow Channel Geometry
- Full Bore, Smooth Transitions: As noted earlier, the ball’s bore matches the pipeline DN, and flow channels have rounded edges (radius ≥1.5 mm) to eliminate dead zones. The ball is designed with a “floating” seat to ensure full contact with the seal—preventing product trapping between the ball and seat.
- No Retention Areas: The valve body has no internal threads, welds, or recesses in contact with the product. Welds (e.g., body seams) are performed via TIG (Tungsten Inert Gas) welding with argon shielding to avoid weld spatter—welds are ground smooth and electrolytically polished to match the surrounding surface finish.
- Minimal Dead Volume: Dead volume (unflushed areas) is calculated using 3D CAD modeling and validated via flow testing—typically ≤0.05 mL per inch of DN for sterile applications.
C. Sealing System Design
- Double Sealing: Many pharmaceutical valves feature a double-sealing system (primary seal between ball and seat, secondary seal between stem and bonnet) to prevent cross-contamination. The stem seal uses a multi-layer design (e.g., PTFE V-rings with EPDM backup rings) to withstand CIP/SIP cycles and maintain leak-tight sealing.
- Sanitary Bonnet Design: The bonnet is bolted (not threaded) to the central body, with a smooth, flush connection to avoid dead zones. The stem is sealed with a diaphragm or bellows in ultra-sterile applications (e.g., WFI systems) to eliminate the risk of stem leakage.
- Seal Retention: Seals are held in place by sanitary retainers (no threads or adhesives) that are easy to remove during maintenance. Retainers are designed to be flush with the valve body, preventing product buildup.
D. Actuation and Control
- Manual Actuation: For small valves (DN≤50), manual actuation uses a handwheel with a torque limiter to prevent over-tightening of the ball against the seat—extending seal life by 30%.
- Automated Actuation: Electric or pneumatic actuators are used for larger valves (DN≥65) or sterile applications where manual contact is minimized. Actuators are IP67-rated (dustproof and waterproof) and compatible with cleanroom environments.
- Position Monitoring: Smart actuators include position sensors (4–20 mA output) to verify valve position (open/closed) for process validation. Some models integrate with PLC systems for automated CIP/SIP sequencing.
V. Technical Specifications of TIANYU 3-Piece Sanitary Ball Valves
TIANYU’s 3-piece stainless steel ball valves are engineered to meet the strictest pharmaceutical requirements, with technical specifications tailored to sterile and non-sterile manufacturing processes.
A. Dimensional and Pressure Ratings
- Nominal Diameter (DN): 15 mm (½”) to 100 mm (4”)—covering common pharmaceutical pipeline sizes (e.g., DN15 for dosing lines, DN100 for WFI distribution).
- Nominal Pressure (PN/Class): PN10 (Class 150) to PN25 (Class 300):
-
- PN10/Class 150: Suitable for low-pressure applications (e.g., formulation tanks, filling lines) with operating pressures up to 1.0 MPa.
-
- PN16/Class 250: Used for medium-pressure applications (e.g., API transfer lines) with pressures up to 1.6 MPa.
-
- PN25/Class 300: Designed for high-pressure applications (e.g., SIP steam lines) with pressures up to 2.5 MPa.
- Face-to-Face Dimensions: Compliant with ISO 5752 (flanged connections) and DIN 3202 (sanitary fittings) to ensure compatibility with existing pharmaceutical equipment.
B. Temperature Range
- Operating Temperature: -20℃ to 180℃ (316L stainless steel, EPDM seals) or -20℃ to 260℃ (316L with PTFE seals).
- CIP Temperature: Up to 95℃ (alkaline/acidic detergents).
- SIP Temperature: Up to 134℃ (steam under pressure, 2.1 MPa) for 30 minutes—compatible with standard pharmaceutical sterilization cycles.
C. Performance Parameters
- Flow Coefficient (KV): 15 (DN15) to 120 (DN100)—full bore design ensures high flow rates (e.g., DN50 valve handles 50 m³/h at ΔP=0.1 MPa).
- Operating Torque: 30 N·m (DN15) to 150 N·m (DN100) for manual operation; automated actuators require torque output ≥150% of the valve’s maximum torque.
- Leakage Rate: ≤0.1 cm³/min for liquid service (ISO 5208 Class VI) and ≤0.1 cm³/min for gas service (helium leak test).
- Cycle Life: 5,000 open/close cycles without seal replacement—equivalent to 10 years of service in typical pharmaceutical operations (2–3 cycles per day).
D. Sanitary Certifications
All TIANYU 3-piece sanitary ball valves hold the following certifications:
- FDA 21 CFR Part 177.2600 (material safety).
- 3A 603-02 (sanitary design).
- ISO 10993-1 (biological evaluation).
- CE (EN 12266-1 for industrial valves).
VI. Manufacturing Process: Ensuring Sanitary Excellence
TIANYU’s manufacturing process for 3-piece stainless steel ball valves adheres to cGMP principles and incorporates strict quality control measures to ensure sanitary performance. The process takes place in a dedicated facility with cleanroom assembly areas and specialized equipment.

A. Raw Material Inspection
- Stainless Steel: Sourced from certified mills (e.g., Outokumpu, Aperam) with Mill Test Certificates (MTCs) verifying chemical composition and mechanical properties. Each batch undergoes spectral analysis to confirm Cr, Ni, and Mo content—critical for corrosion resistance.
- Seals and Polymers: EPDM/PTFE seals are sourced from FDA-approved suppliers (e.g., Parker Hannifin) and tested for extractables and SIP compatibility before use.
- Fasteners: 316L stainless steel bolts and nuts are used to avoid galvanic corrosion—fasteners are passivated and cleaned before assembly.
B. Precision Machining
- Valve Body and End Caps: Machined using 5-axis CNC lathes with dimensional tolerances of ±0.02 mm. Flow channels are mechanically polished to Ra ≤0.8 μm before electrolytic polishing.
- Ball and Stem: The ball is turned and milled to a DN-matching bore, then electrolytically polished to Ra ≤0.38 μm. The stem is ground to a surface finish of Ra ≤0.4 μm and a concentricity tolerance of 0.01 mm to ensure smooth operation and sealing.
- Welding: TIG welding is used for all body seams and component attachments. Welds are performed in a controlled environment (argon shielding) to avoid oxidation and weld spatter. Post-welding, welds are ground smooth and electrolytically polished to match the surrounding surface.
C. Cleanroom Assembly
- Cleanroom Classification: Assembly takes place in a Class 10,000 (ISO 14644-1 Class 7) cleanroom to prevent particulate contamination. Workers wear sterile gowns, gloves, and face masks—airborne particle counts are monitored continuously (≤10,000 particles/m³ ≥0.5 μm).
- Assembly Process: Components are cleaned with deionized water and isopropyl alcohol before assembly. Seals are installed using sterile tools to avoid damage. The valve is cycled 10 times to verify smooth operation, and torque is measured at each cycle to ensure consistency.
- Final Cleaning: After assembly, valves are flushed with WFI (Water for Injection) to remove any residual particles—total particulate count in the flush water must be ≤10 particles/mL ≥0.5 μm.
D. Quality Control and Testing
- Surface Finish Testing: A profilometer measures Ra on 5 key contact surfaces per valve—Ra must be ≤0.38 μm. Visual inspection under 10x magnification checks for scratches, pits, or tool marks.
- Pressure Testing: Each valve undergoes a hydrostatic test (1.5x PN for 30 minutes) and a pneumatic test (1.1x PN for 15 minutes) to verify leak-tight sealing. For sterile applications, a helium leak test (leak rate ≤1×10⁻⁹ Pa·m³/s) is performed.
- CIP/SIP Validation: Sample valves (1 per production batch) undergo 1,000 CIP/SIP cycles—post-test, seals are inspected for degradation, and surface finish is re-verified.
- Microbial Testing: Valves are inoculated with E. coli and Staphylococcus aureus, then subjected to a SIP cycle. Post-SIP, no viable microorganisms are detected (SAL 10⁻⁶).
E. Packaging and Documentation
- Packaging: Valves are packaged in sterile, moisture-resistant bags (FDA-approved) and labeled with the serial number, manufacturing date, and certification marks.
- Documentation: Each valve is accompanied by a Certificate of Compliance (CoC) and a Batch Record, including:
-
- Raw material MTCs.
-
- Surface finish test results.
-
- Pressure and leak test reports.
-
- CIP/SIP validation data.
-
- Certification copies (FDA, 3A, ISO).
VII. Real-World Applications in Pharmaceutical Manufacturing
TIANYU’s 3-piece stainless steel ball valves are used across all stages of pharmaceutical production—from API synthesis to sterile filling. Below are three detailed case studies highlighting their performance and value.
A. Case Study 1: API Manufacturing Plant (India)
- Challenge: A large API manufacturer producing antibiotics faced frequent valve failures due to corrosion from acidic intermediates (pH 2–3) and difficulty cleaning viscous product residue. Maintenance downtime was 8 hours/week, and batch rejection rates were 2.5% due to cross-contamination.
- Solution: TIANYU 3-piece 316L ball valves (DN25–DN50, PN16) with EPDM seals and electrolytically polished surfaces. The 3-piece design allowed in-line maintenance, and the 316L material resisted acid corrosion.
- Results:
-
- Maintenance downtime reduced by 75% (from 8 to 2 hours/week) due to easy disassembly.
-
- Batch rejection rate dropped to 0.3% (a 88% improvement) due to improved cleaning and sealing.
-
- Valve lifespan extended from 2 to 8 years—reducing replacement costs by $120,000/year.
B. Case Study 2: Sterile Injectable Filling Line (Germany)
- Challenge: A biopharmaceutical company producing sterile injectables needed valves compatible with WFI (Water for Injection) and SIP cycles (121℃, 30 minutes). The existing two-piece valves required pipeline removal for maintenance, causing 4 hours of downtime per cleaning cycle.
- Solution: TIANYU 3-piece 316Ti ball valves (DN15–DN32, PN25) with PTFE seals and bellows stem seals. The 3-piece design allowed in-line cleaning and maintenance, and the 316Ti material withstood repeated SIP cycles.
- Results:
-
- Maintenance downtime reduced by 80% (from 4 to 0.8 hours per cycle).
-
- SIP validation time cut by 30% (from 2 to 1.4 hours) due to improved flow channel geometry.
-
- No microbial contamination detected in 2 years of operation—achieving consistent SAL 10⁻⁶.
C. Case Study 3: Pharmaceutical Water System (U.S.)
- Challenge: A municipal pharmaceutical contract manufacturer needed valves for its PW (Purified Water) and WFI distribution systems. The valves had to meet 3A standards, withstand 95℃ CIP cycles, and have a Ra ≤0.38 μm surface finish.
- Solution: TIANYU 3-piece 316L ball valves (DN50–DN100, PN16) with EPDM seals and full bore design. The valves were equipped with smart actuators for position monitoring and automated CIP sequencing.
- Results:
-
- Water system validation passed on the first attempt (FDA inspection compliant).
-
- CIP cycle time reduced by 25% (from 60 to 45 minutes) due to full bore flow channels.
-
- Annual maintenance costs reduced by $45,000 compared to the previous valve supplier.
VIII. Maintenance Best Practices for Sanitary Performance
Proper maintenance is critical to ensuring 3-piece stainless steel ball valves maintain their sanitary performance and regulatory compliance over time. Below are industry-proven best practices tailored to pharmaceutical applications.

A. Regular Cleaning and Sterilization
- CIP Parameters:
-
- Detergent: Alkaline (0.5–2% NaOH) or acidic (1–3% HNO₃) detergents approved for pharmaceutical use.
-
- Temperature: 80–95℃ for 15–30 minutes—ensures complete removal of product residue.
-
- Flow Rate: 1–2 m/s through the valve to create turbulent flow (critical for removing adhering residue).
- SIP Parameters:
-
- Temperature: 121℃ (1.0 MPa) for 30 minutes or 134℃ (2.1 MPa) for 15 minutes—follows ISO 11134 (sterilization of medical devices).
-
- Steam Quality: Dry saturated steam (moisture content ≤5%) to avoid water spotting on contact surfaces.
- Cleaning Verification:
-
- Visual inspection: Use a borescope to check for residue in flow channels—no visible residue allowed.
-
- Microbiological testing: Swab contact surfaces and incubate for 24 hours—microbial count ≤10 CFU/cm².
-
- Chemical testing: Measure total organic carbon (TOC) in flush water—TOC ≤500 μg/L.
B. Routine Inspection and Component Replacement
- Inspection Frequency:
-
- Daily: Visual check for external leakage and actuator functionality.
-
- Weekly: Verify valve position and torque (manual valves) or actuator calibration (automated valves).
-
- Quarterly: Disassemble and inspect internal components (ball, seat, stem) for wear or corrosion.
- Component Replacement Schedules:
-
- Seals: Replace EPDM seals every 18–24 months or after 500 SIP cycles; replace PTFE seals every 24–36 months.
-
- Ball/Stem: Replace if surface finish degrades to Ra >0.5 μm or if pitting/corrosion is detected (depth >0.1 mm).
-
- Fasteners: Replace every 3 years to prevent galvanic corrosion.
C. Storage and Handling
- Cleanroom Storage: Store spare valves and components in a Class 10,000 cleanroom to prevent particulate contamination.
- Moisture Control: Keep valves dry (relative humidity ≤60%) to avoid corrosion during storage.
- Avoid Contamination: Do not touch contact surfaces with bare hands—use sterile gloves and tools during installation and maintenance.
D. Documentation and Validation
- Maintenance Records: Document all maintenance activities (cleaning, inspection, component replacement) with dates, personnel, and test results—required for FDA/EMA audits.
- Requalification: After major maintenance (e.g., seal replacement), requalify the valve for CIP/SIP compatibility and leak tightness.
- Calibration: Calibrate automated actuators and position sensors every 6 months to ensure accurate operation.
IX. TIANYU’s Customization Advantages for Pharmaceutical Valves
TIANYU understands that pharmaceutical manufacturers have unique process requirements—from specialized media compatibility to custom flow control. TIANYU’s 3-piece stainless steel ball valves offer extensive customization options to meet these needs, while maintaining regulatory compliance and sanitary performance.

A. Material Customization
- Corrosion-Resistant Alloys: For aggressive media (e.g., strong acids, chlorinated detergents), TIANYU offers Hastelloy C-276 or Alloy 28 valves—corrosion rate ≤0.005 mm/year.
- Low-Leach Materials: For ultra-pure applications (e.g., biopharmaceuticals), TIANYU provides 316L valves with low-leach seals (total extractables ≤5 μg/mL) and electropolished surfaces (Ra ≤0.25 μm).
- Temperature-Resistant Seals: For high-temperature SIP (134℃) or low-temperature storage (-40℃), TIANYU offers custom seal materials (e.g., perfluoroelastomers for 200℃+ service).
B. Design Customization
- Flow Channel Optimization: For viscous products (e.g., ointments, suspensions), TIANYU modifies the ball bore and flow channels to reduce pressure loss—KV improved by 15% compared to standard designs.
- Sterile Connections: Custom end connections (e.g., tri-clamp, weld-in, or flanged) to match existing pharmaceutical equipment—tri-clamp connections comply with 3A 603-02.
- Actuation Integration: Tailored actuators (electric, pneumatic, or manual) with custom torque ratings, position feedback, and PLC compatibility for automated process control.
- Ultra-Sanitary Features: For sterile manufacturing (e.g., injectables), TIANYU offers bellows-sealed stems (eliminating stem leakage) and dead-volume-free designs (≤0.02 mL per inch of DN).
C. Compliance and Validation Support
- Custom Documentation: TIANYU provides detailed validation packages, including:
-
- Design History File (DHF) and Device Master Record (DMR) per FDA 21 CFR Part 820.
-
- CIP/SIP validation protocols and test reports.
-
- Material compatibility data (extractables/leachables, microbial growth).
- Audit Support: TIANYU’s quality team assists with FDA/EMA audits, providing access to manufacturing records, test data, and certification documents.
D. Service and Support
- Pre-Sales Engineering: A team of pharmaceutical valve experts (with 10+ years of industry experience) works with customers to define requirements, select materials, and optimize valve design.
- On-Site Installation and Training: Global service engineers (response time ≤48 hours) provide on-site installation guidance, CIP/SIP training, and maintenance workshops.
- Warranty: 2-year standard warranty, with optional 3-year extended warranty for critical applications—covers material defects, leakage, and performance issues.
TIANYU’s 3-Piece Sanitary Ball Valves—Purity and Compliance for Pharma
TIANYU’s 3-piece stainless steel ball valves deliver tailored sanitary solutions for pharmaceutical manufacturing, blending 316L/316Ti’s corrosion resistance, Ra ≤0.38 μm surfaces, and CIP/SIP compatibility to meet FDA/3A standards. Customizable designs (flow channels, seals, connections) solve unique process challenges, from API synthesis to sterile filling. With 5,000-cycle durability, 80% reduced maintenance downtime, and \(45k–\)120k annual cost savings (per case study), these valves ensure product purity and regulatory compliance. Backed by validation support, 48-hour global service, and 2–3-year warranties, TIANYU’s valves are the trusted choice for pharmaceutical manufacturers prioritizing safety, efficiency, and customization.