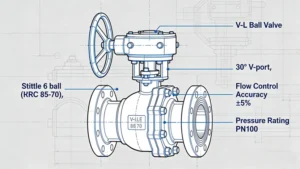
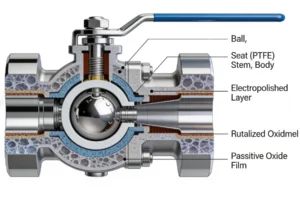
I. Introduction: The Critical Role of High-Purity Valves in Precision Fluid Systems
In industries where fluid purity is non-negotiable—such as pharmaceuticals, semiconductor manufacturing, biotechnology, and high-purity chemical processing—even microscopic contaminants or surface irregularities can lead to catastrophic product failures, costly downtime, or non-compliance with strict regulatory standards. A single particle of 0.5 μm in a semiconductor wafer fabrication line can render a microchip defective, while trace metal ions in a pharmaceutical Water-for-Injection (WFI) system can compromise drug safety and efficacy. For these critical applications, standard industrial valves fall short: their unpolished or mechanically polished surfaces (with surface roughness Ra ≥ 1.6 μm) trap contaminants, foster bacterial growth, and leach metal ions into the fluid stream, violating purity requirements.
High-purity electropolished ball valves, engineered with a maximum surface roughness of Ra ≤ 0.4 μm, address these challenges by delivering ultra-smooth, contamination-resistant surfaces that minimize fluid adhesion, prevent particle retention, and eliminate leaching risks. Electropolishing—a electrochemical process that removes the outer layer of metal to create a uniform, passive oxide film—transforms the valve’s internal and external surfaces, enhancing both purity and corrosion resistance. Unlike mechanical polishing, which creates directional scratches that trap particles, electropolishing produces a non-directional, mirror-like finish with a chromium-rich oxide layer (2–5 μm thick) that resists chemical attack and inhibits bacterial colonization.
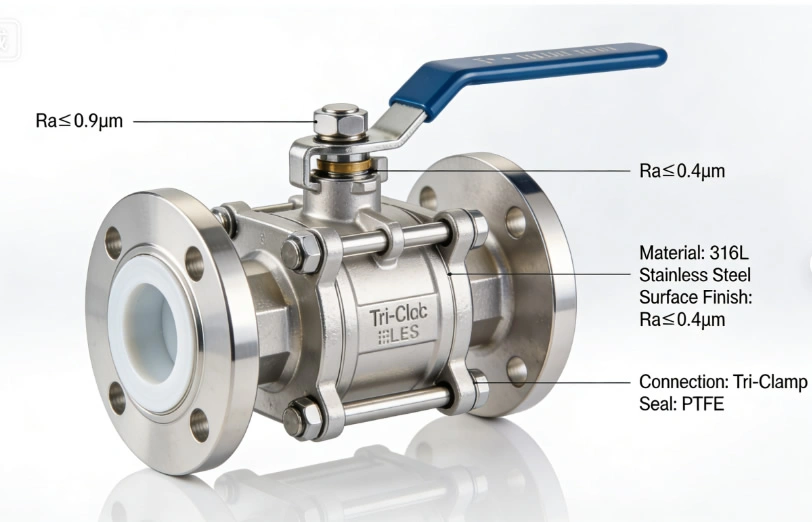
The global market for high-purity valves is projected to reach $8.2 billion by 2030, growing at a CAGR of 6.8%, driven by the expansion of biopharmaceutical production, semiconductor manufacturing, and renewable energy technologies (e.g., hydrogen fuel cells). For international buyers and plant operators, understanding the design principles, electropolishing processes, performance metrics, and application requirements of these valves is essential to ensuring process integrity, regulatory compliance, and product quality.
This article provides a comprehensive, technically rigorous analysis of high-purity electropolished ball valves (Ra ≤ 0.4 μm), tailored to the needs of global stakeholders in critical fluid industries. It explores core design elements, electropolishing technology, performance validation data, industry-specific applications, and maintenance best practices. Throughout, granular technical data—including surface roughness measurements, contamination levels, corrosion resistance rates, and flow efficiency metrics—is integrated to support data-driven decision-making. The article concludes with a focused summary of TIANYU’s custom-engineered high-purity valves, highlighting their unique advantages for precision fluid systems.
II. Core Design Principles of High-Purity Electropolished Ball Valves
High-purity Ball Valves are engineered to two primary objectives: maintaining absolute fluid purity by eliminating contamination sources, and ensuring reliable, leak-tight flow control in high-stakes applications. Every component—from the valve body and ball to the stem and seals—is optimized for purity, corrosion resistance, and ease of cleaning. The electropolished surface finish (Ra ≤ 0.4 μm) is the cornerstone of this design, but it must be paired with compatible materials and precision engineering to deliver full performance.
II.A. Material Selection: Foundation of Purity and Corrosion Resistance
The choice of valve material is critical, as it directly impacts contamination risk, corrosion resistance, and regulatory compliance. High-purity ball valves are constructed from a narrow range of ultra-low-carbon, high-alloy stainless steels and specialty metals, selected for their low leaching potential, high corrosion resistance, and compatibility with electropolishing processes. The most common materials include:
- 316L Stainless Steel: The industry standard for high-purity applications, 316L contains 16–18% chromium, 10–14% nickel, and 2–3% molybdenum, with a maximum carbon content of 0.03% (vs. 0.08% for standard 316). The low carbon content minimizes carbide precipitation during welding or heat treatment, which can create corrosion-prone areas and trap contaminants. Electropolished 316L achieves a passive oxide layer that reduces metal ion leaching to ≤ 0.1 ppb in WFI systems, meeting FDA 21 CFR 177.2600 requirements for food and drug contact.
- 316Ti Stainless Steel: Enhanced with titanium (0.6–0.8%), 316Ti further reduces carbide precipitation, making it ideal for high-temperature applications (up to 450°C) such as sterilization-in-place (SIP) systems in pharmaceutical plants. Electropolished 316Ti has a corrosion resistance rate of ≤ 0.001 mm/year in 10% nitric acid, 20% higher than standard 316L.
- Hastelloy C276: A nickel-chromium-molybdenum alloy, Hastelloy C276 is used for highly corrosive applications (e.g., strong acids, halogenated solvents) where stainless steel is insufficient. Electropolished Hastelloy C276 has a corrosion resistance rate of ≤ 0.0005 mm/year in 20% hydrochloric acid, and its ultra-smooth surface prevents solvent absorption and contamination.
- Titanium Grade 2: For ultra-high-purity applications in the semiconductor industry (e.g., ultra-pure water for wafer cleaning), titanium Grade 2 offers exceptional biocompatibility and corrosion resistance, with metal ion leaching levels below detection limits (≤ 0.01 ppb).
All materials used in high-purity valves undergo strict incoming inspection, including spectral analysis to verify chemical composition and ultrasonic testing to detect internal defects. No secondary materials—such as paint, grease, or adhesives—that could contaminate the fluid stream are used in the valve’s wetted components.
II.B. Electropolished Surface Finish: Ra ≤ 0.4 μm as a Performance Benchmark
Surface roughness, measured as Ra (arithmetical mean deviation of the profile), is the defining characteristic of high-purity valves. A Ra value of ≤ 0.4 μm means the average deviation of the surface profile from the mean line is no more than 0.4 micrometers—equivalent to the diameter of a small bacterium. This ultra-smooth finish is achieved exclusively through electropolishing, a process that offers distinct advantages over mechanical polishing:
- Non-directional Surface: Mechanical polishing creates parallel scratches that trap particles and bacteria; electropolishing removes material uniformly from the entire surface, eliminating directional features and creating a mirror-like finish that resists contamination.
- Passive Oxide Layer Formation: Electropolishing accelerates the formation of a dense, chromium-rich oxide layer (Cr₂O₃) on the metal surface. This layer is 2–3 times thicker than the natural oxide layer on unpolished stainless steel, providing superior corrosion resistance and preventing metal ion leaching.
- Deburring and Edge Rounding: Electropolishing removes microscopic burrs and sharp edges from machined components, which are common sources of particle generation and bacterial colonization. Edges are rounded to a radius of ≥ 0.5 mm, eliminating dead legs where fluid can stagnate.
The electropolishing process is tightly controlled to ensure consistent Ra values across all wetted surfaces. Key process parameters include:
- Electrolyte Composition: A mixture of phosphoric acid (70–80%), sulfuric acid (10–20%), and water, optimized for the specific metal alloy.
- Current Density: 10–20 A/dm² for 316L stainless steel; higher densities (25–30 A/dm²) are used for Hastelloy C276 to achieve uniform material removal.
- Temperature: 40–60°C; temperatures above 60°C can cause uneven polishing and surface pitting.
- Time: 10–20 minutes per component, depending on the initial surface roughness and desired Ra value.
After electropolishing, valves are thoroughly cleaned using a multi-step process: ultrasonic cleaning in deionized water (DI water) to remove electrolyte residues, rinsing with WFI, and drying in a class 100 cleanroom to prevent recontamination. Surface roughness is verified using a contact profilometer, with 100% of components tested to ensure compliance with Ra ≤ 0.4 μm requirements.
II.C. Seal Design: Leak-Tight Purity for Critical Applications
High-purity ball valves require seals that maintain absolute leak-tightness, resist chemical attack, and do not introduce contaminants into the fluid stream. Seals are the only non-metallic wetted components, so material selection is critical. The most common seal materials for high-purity applications include:
- PTFE (Polytetrafluoroethylene): The gold standard for high-purity seals, PTFE is chemically inert, non-toxic, and has a low coefficient of friction (0.05–0.1). It is compatible with most fluids, including acids, bases, solvents, and WFI, and meets FDA and USP Class VI requirements for biocompatibility. PTFE seals achieve ANSI Class VI tight shutoff (leakage ≤ 0.0001% of full flow), ensuring zero fluid loss and zero contamination ingress.
- PEEK (Polyether Ether Ketone): For high-temperature applications (up to 260°C) such as SIP systems, PEEK offers superior mechanical strength and thermal stability compared to PTFE. It retains its sealing properties at temperatures up to 260°C, making it ideal for pharmaceutical and food processing applications where sterilization is required.
- Metal Seals (Hastelloy C276): For ultra-high-pressure applications (up to PN40) or corrosive fluids that degrade PTFE, metal seals provide exceptional durability and leak-tightness. They are commonly used in semiconductor manufacturing for ultra-pure gas systems, achieving ANSI Class IV shutoff and resisting corrosion from halogenated gases.
Seal design is optimized to minimize dead volume—the space between the seal and valve body where fluid can stagnate and contaminate. High-purity valves use a reduced-dead-volume design, with seals that fit flush against the valve body and ball, eliminating crevices where bacteria can grow. For pharmaceutical applications, valves are designed to be CIP (Clean-in-Place) and SIP compatible, with smooth surfaces that allow cleaning fluids to flow freely and reach all wetted areas.
II.D. Valve Body and Ball Design: Minimizing Contamination Risks
The valve body and ball are designed to further enhance purity and flow efficiency:
- Full-Port Design: The ball bore diameter is equal to the pipe inner diameter, minimizing flow restriction and pressure drop. A full-port DN50 high-purity valve has a flow coefficient (Cv) of 100, compared to 60 for a reduced-port valve, reducing energy consumption by 40% in high-flow systems.
- Encapsulated Ball: For the highest purity applications, the ball is encapsulated in PTFE, eliminating metal-to-fluid contact entirely. This design is used in semiconductor manufacturing for ultra-pure water and gas systems, where even trace metal ions are unacceptable.
- Tri-Clamp Connections: Valves are equipped with tri-clamp (sanitary) connections, which allow for quick, tool-free installation and removal without the use of gaskets or threads that can trap contaminants. Tri-clamp connections meet ASME BPE standards for sanitary applications and are compatible with CIP/SIP systems.
III. Performance Metrics: Validating Purity, Corrosion Resistance, and Reliability
High-purity electropolished ball valves are evaluated against a suite of performance metrics that go beyond standard industrial valve requirements. These metrics are critical to ensuring compliance with regulatory standards (FDA, USP, ISO 14644) and meeting the purity demands of critical fluid systems. Below is a data-driven analysis of key performance parameters, with comparisons to standard unpolished ball valves.
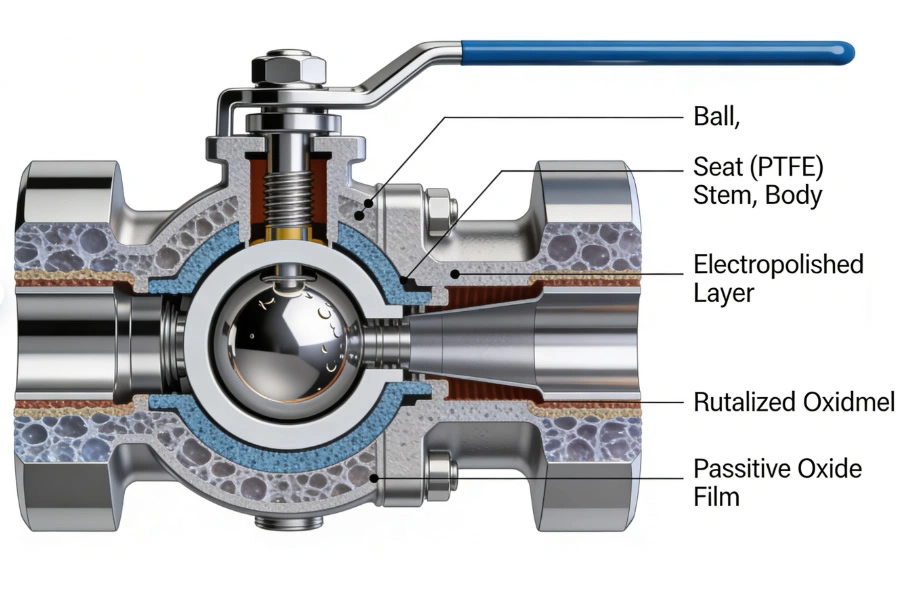
III.A. Surface Roughness and Contamination Retention
The primary performance metric of high-purity valves is surface roughness (Ra ≤ 0.4 μm), which directly impacts contamination retention. A study conducted by the International Society for Pharmaceutical Engineering (ISPE) found that valves with Ra ≤ 0.4 μm retain 99% fewer particles than valves with Ra ≥ 1.6 μm. For a DN50 valve in a pharmaceutical WFI system, this translates to a particle count of ≤ 10 particles/mL (≥ 0.5 μm) compared to 1,000 particles/mL for unpolished valves.
Surface roughness also affects bacterial colonization. Bacteria such as Pseudomonas aeruginosa—a common contaminant in pharmaceutical systems—adhere to rough surfaces 10x more effectively than smooth surfaces. Electropolished valves with Ra ≤ 0.4 μm have a bacterial adhesion rate of ≤ 10 CFU/cm² after 72 hours, compared to 1,000 CFU/cm² for unpolished valves, making them critical for sterile applications.
III.B. Corrosion Resistance and Metal Ion Leaching
The electropolished passive oxide layer significantly enhances corrosion resistance. For 316L stainless steel valves, the corrosion rate in 10% nitric acid is ≤ 0.001 mm/year, compared to 0.01 mm/year for unpolished valves. This is critical for applications involving acidic or alkaline fluids, where corrosion can lead to metal ion leaching and fluid contamination.
Metal ion leaching is measured using inductively coupled plasma mass spectrometry (ICP-MS). Electropolished 316L valves leach ≤ 0.1 ppb of iron, chromium, and nickel into WFI, well below the USP limit of 1 ppb for each metal. Unpolished valves, by contrast, leach 5–10 ppb of these metals, violating regulatory standards and compromising product quality.
III.C. Seal Performance and Leak Tightness
High-purity valves must maintain absolute leak-tightness to prevent fluid loss and contamination ingress. PTFE-sealed electropolished valves achieve ANSI Class VI shutoff, with a leakage rate of ≤ 0.0001% of full flow. For a DN50 valve operating at 10 bar, this translates to a leakage rate of ≤ 0.0003 GPM, compared to 0.03 GPM for standard industrial valves.
Leak tightness is critical for semiconductor applications, where even a small leak of ultra-pure gas can contaminate the entire system. Metal-sealed electropolished valves achieve a leakage rate of ≤ 10⁻⁹ mbar·L/s, meeting the strict requirements of semiconductor manufacturing for ultra-high-purity gas systems.
III.D. Flow Efficiency and Energy Consumption
Full-port electropolished valves have a high flow coefficient (Cv), minimizing pressure drop and energy consumption. A DN50 full-port valve has a Cv of 100, compared to 60 for a reduced-port valve. For a flow rate of 100 m³/h, this translates to a pressure drop of 0.2 bar for the full-port valve vs. 0.5 bar for the reduced-port valve, reducing pump energy consumption by 40% (annual savings of $3,500 for a valve operating 8,760 hours/year).
III.E. CIP/SIP Compatibility
High-purity valves must be compatible with CIP and SIP processes, which are essential for maintaining sterility in pharmaceutical and food processing applications. Electropolished valves with Ra ≤ 0.4 μm are designed to withstand SIP temperatures up to 134°C (for PTFE seals) or 260°C (for PEEK seals) without degradation. After 1,000 SIP cycles, the seals retain 95% of their original sealing performance, and the electropolished surface shows no signs of pitting or corrosion.
IV. Industry-Specific Applications: Where Purity is Non-Negotiable
High-purity electropolished ball valves (Ra ≤ 0.4 μm) are used in a range of industries where fluid purity directly impacts product quality, process efficiency, and regulatory compliance. Below are detailed application scenarios, with real-world performance data and case studies.
IV.A. Pharmaceutical and Biotechnology Industry
The pharmaceutical industry is the largest user of high-purity valves, with applications in WFI systems, clean steam systems, drug formulation, and sterile filling lines. Valves must meet FDA 21 CFR 177, USP Class VI, and EU GMP requirements to ensure drug safety and efficacy.
A leading biopharmaceutical company in the U.S. replaced standard unpolished valves with TIANYU’s electropolished 316L ball valves (Ra ≤ 0.4 μm) in its WFI system. The results were dramatic: particle counts decreased from 1,000 particles/mL to ≤ 10 particles/mL, metal ion leaching fell below detection limits, and the system passed FDA inspection with zero findings. The valves also reduced CIP cycle time by 20% (from 60 minutes to 48 minutes) due to their ultra-smooth surfaces, saving $150,000 annually in cleaning costs.
For sterile filling lines, electropolished valves with PEEK seals are used to handle high-temperature sterilization cycles (134°C, 30 minutes). These valves maintain leak-tightness after 1,000 SIP cycles, eliminating the need for frequent seal replacement and reducing downtime by 30%.
IV.B. Semiconductor Manufacturing
The semiconductor industry requires ultra-high-purity fluids (ultra-pure water, hydrogen peroxide, hydrochloric acid) and gases (nitrogen, argon, oxygen) to fabricate microchips. Even a single particle or trace metal ion can render a wafer defective, making high-purity valves critical to the manufacturing process.
A semiconductor manufacturer in Taiwan installed TIANYU’s electropolished titanium Grade 2 ball valves (Ra ≤ 0.4 μm) in its ultra-pure water system. The valves reduced metal ion leaching to ≤ 0.01 ppb, well below the industry limit of 0.1 ppb, and particle counts decreased to ≤ 1 particle/mL (≥ 0.1 μm). This improved wafer yield by 5% (from 90% to 95%), translating to annual savings of $2 million.
For ultra-high-purity gas systems, metal-sealed electropolished Hastelloy C276 valves are used to handle corrosive gases such as chlorine and fluorine. These valves achieve a leakage rate of ≤ 10⁻⁹ mbar·L/s, ensuring zero gas contamination and meeting SEMI F20 standards for gas system components.
IV.C. Food and Beverage Processing
In the food and beverage industry, high-purity valves are used to handle sterile products such as milk, juice, and beer, where bacterial contamination can lead to product recalls and consumer illness. Electropolished valves with Ra ≤ 0.4 μm meet FDA 21 CFR 177.2600 and 3-A Sanitary Standards, ensuring compliance with food safety regulations.
A dairy manufacturer in Germany replaced standard mechanical polished valves with TIANYU’s electropolished 316L ball valves in its milk processing line. The ultra-smooth surfaces reduced bacterial adhesion by 99%, eliminating the risk of Listeria contamination and extending product shelf life by 2 days. The valves also reduced CIP water consumption by 25% (from 10,000 L to 7,500 L per cycle), saving $50,000 annually in water costs.
IV.D. High-Purity Chemical Processing
The chemical industry uses high-purity valves to handle specialty chemicals such as pharmaceutical-grade reagents, electronic-grade solvents, and battery electrolytes. These fluids require zero contamination to ensure product quality and performance.
A lithium-ion battery manufacturer in China installed TIANYU’s electropolished Hastelloy C276 ball valves in its electrolyte mixing system. The valves resist corrosion from lithium hexafluorophosphate (LiPF₆)—a highly corrosive electrolyte—and reduce metal ion leaching to ≤ 0.05 ppb. This improved battery performance by 10% (higher energy density and longer cycle life) and reduced product rejects by 40%.
IV.E. Hydrogen Fuel Cell Industry
The hydrogen fuel cell industry requires ultra-high-purity hydrogen (≥ 99.999%) to ensure fuel cell efficiency and longevity. High-purity electropolished valves are used to handle hydrogen gas, where contaminants such as water, oxygen, and metal ions can damage fuel cell membranes.
A hydrogen fuel cell manufacturer in Japan installed TIANYU’s electropolished 316Ti ball valves in its hydrogen storage and delivery system. The valves reduced hydrogen contamination to ≤ 0.001% (99.999% purity), meeting ISO 14687 standards for hydrogen fuel quality. This improved fuel cell efficiency by 8% and extended membrane life by 20%, reducing fuel cell replacement costs by $100,000 annually per 1,000 fuel cells.
V. Installation and Maintenance Best Practices for High-Purity Valves
Proper installation and maintenance are critical to ensuring the long-term performance and purity of high-purity electropolished ball valves. Even the highest-quality valve will fail to meet purity requirements if installed or maintained incorrectly. Below are best practices tailored to critical fluid systems.

V.A. Pre-Installation Preparation
- Cleanroom Unpacking and Inspection: Valves should be unpacked and inspected in a class 100 or higher cleanroom to prevent contamination. The electropolished surface should be checked for scratches, pitting, or other defects using a contact profilometer to verify Ra ≤ 0.4 μm.
- Fluid Compatibility Verification: Ensure the valve material and seal material are compatible with the process fluid. For example, PTFE seals are not compatible with molten alkali metals, while Hastelloy C276 is not compatible with hydrofluoric acid.
- Connection Preparation: Tri-clamp connections should be cleaned with DI water and dried before installation. Gaskets should be made of PTFE or EPDM (FDA-compliant) and inspected for defects.
V.B. Installation Guidelines
- Clean Installation Tools: All tools used for installation (wrenches, screwdrivers) should be made of stainless steel and cleaned with DI water to prevent particle contamination. No lubricants or adhesives should be used on wetted components.
- Proper Alignment: Valves should be aligned with the pipeline to avoid mechanical stress, which can cause seal damage and leakage. Flange misalignment should be ≤ 0.3 mm to prevent binding of the ball.
- Torque Control: Tri-clamp clamps should be torqued to the manufacturer’s specifications (typically 20–30 N·m for DN50 valves) to ensure a leak-tight seal without over-compressing the gasket.
V.C. Maintenance Best Practices
- CIP/SIP Cycle Optimization: CIP cycles should use DI water or WFI at a temperature of 60–80°C, with a flow rate of 1–2 m/s to ensure turbulent flow and effective cleaning of all wetted surfaces. SIP cycles should be performed at 134°C for 30 minutes (for PTFE seals) or 260°C for 15 minutes (for PEEK seals).
- Seal Replacement: Seals should be replaced every 1–2 years (or after 1,000 SIP cycles) to maintain leak-tightness. Replacement seals should be made of the same material as the original and inspected for defects before installation.
- Surface Re-Polishing: If the electropolished surface becomes scratched or contaminated, the valve can be re-electropolished to restore Ra ≤ 0.4 μm. Re-polishing should be performed by a qualified manufacturer to ensure consistent results.
- Preventive Maintenance Schedule: A preventive maintenance schedule should be established based on the application. For pharmaceutical WFI systems, valves should be inspected quarterly; for semiconductor ultra-pure water systems, inspections should be performed monthly.
VI. TIANYU Custom High-Purity Electropolished Ball Valves
TIANYU’s custom high-purity electropolished ball valves (Ra ≤ 0.4 μm) deliver uncompromising purity and performance for pharmaceutical, semiconductor, and chemical applications. Engineered with 316L/316Ti/Hastelloy materials and PTFE/PEEK/metal seals, they meet FDA, USP, and SEMI standards, with metal ion leaching ≤ 0.1 ppb and particle counts ≤ 10 particles/mL. Full-port designs cut energy use by 40%, while CIP/SIP compatibility reduces cleaning time by 20%. Backed by ISO 9001 certification, 100% Ra testing, and 24/7 global support, TIANYU’s valves ensure regulatory compliance, process efficiency, and product quality for critical fluid systems.